Could someone please help explain how they got to this next step (writing in tabular form) in solving this chemical equation? (I have the worked answer, but I don't understand the first step they did).
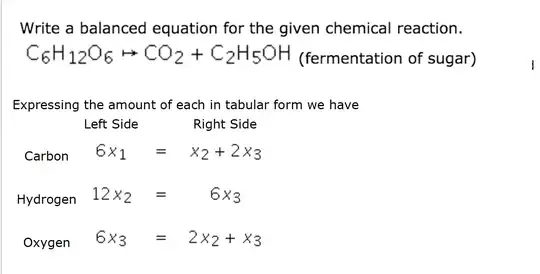
Could someone please help explain how they got to this next step (writing in tabular form) in solving this chemical equation? (I have the worked answer, but I don't understand the first step they did).

If we are to balance the equation, that means finding positive integers $x_1,x_2,x_3$ such that $$ x_1\mathrm{C_6H_{12}O_6}\to x_2\mathrm{CO_2}+x_3\mathrm{C_2H_5OH} $$ has as many of each atom on the left side as it does on the right side.
Looking at carbon first, the number of carbon atoms on the left side is $6x_1$, while on the right side it's $x_2+2x_3$. These two numbers are supposed to be equal, so we put $=$ between them, and that's the first equation.
The two other equations are done similarly for hydrogen and oxygen. However, there is a typo in your picture: it's supposed to be $x_1$ on the left side of all three equations. That is, after all, how many sugar molecules there are on the left-hand side. Thus we get $$ \begin{array}{lccc} \text{Element} &\text{Left side} &&\text{Right side}\\ \text{Carbon}&6x_1&=&x_2+2x_3\\ \text{Hydrogen} &12x_1&=&6x_3\\ \text{Oxygen} &6x_1&=&2x_2+x_3 \end{array} $$